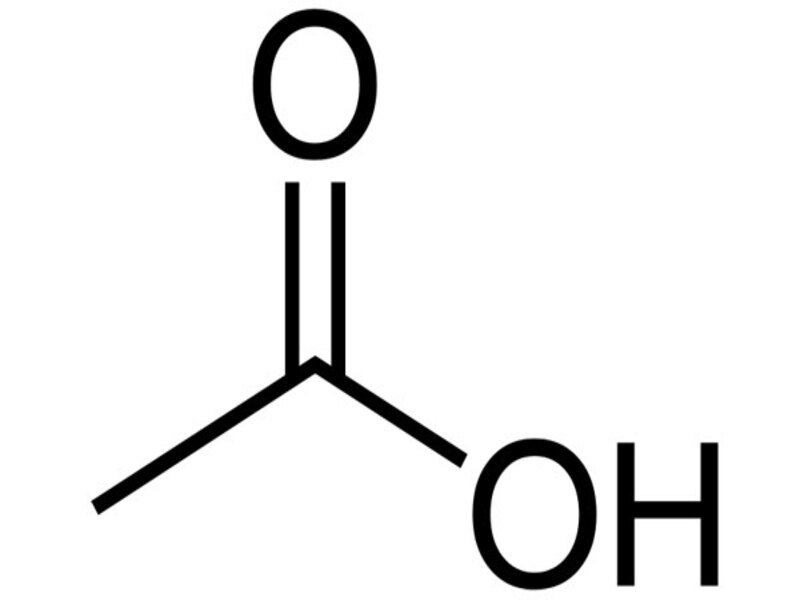
Acetic acid, also known as ethanoic acid, is an organic acid with the formula CH3COOH. It is the organic substance that gives vinegar its sour taste and sharp smell. Vinegar contains 4-5% acetic acid. It is one of the smallest two-carbon carboxylic acids. (The smallest is formic acid). It consists of two small functional groups, one acetyl group and one hydroxyl group. It is formed by the oxidation of carbohydrates in nature. In the industrial industry, acetic acid is obtained by both biological processes and synthetic processes. Acetic acid is called acetate of salt and ester. It is completely soluble in water.
Apart from household vinegar, acetic acid is mostly used as an additive in the production of polyvinyl acetate and cellulose acetate in the industry.
Acetic acid is an important chemical catalyst and industrial chemical, first used in cellulose acetate processes for photographic film, for wood glue, and in the polyvinyl acetate process for synthetic fibers and fabrics.
Acetic acid Acetic acid is widely used in the chemical sector, used as a raw material in the production processes of many chemicals. Its most important use is in the vinyl acetate production process. The resulting polyvinyl acetate is used as wood glue.
Acetic acid The reaction is carried out in the gas phase with palladium catalyst, consisting of oxygen, ethylene, and acetic acid.
2 H3C-COOH + 2 C2H4 + 02 → 2 H3C-CO-O-CH = CH2 + 2H20
Acetic acid Vinyl acetate can be polymerized into polyvinyl acetate, which is a component of paints and adhesives, or into other polymers.
This is followed by the production of acetic anhydride and acetic esters.
Esters of acetic acid are often used as solvents for inks, paints and coatings. Esters include n-butyl acetate, ethyl acetate, isobutyl acetate and propyl acetate. They are typically produced by catalyzed reactions of acetic acid and the corresponding alcohol.
H3C-COOH + HO-R → H3C-CO-O-R + H2O, (R = a general alkyl group)
Acetic acid Also, most acetate esters are obtained from acetaldehyde by the Tishchenko reaction. They are also used as solvents for nitrocellulose, ether acetates, acrylic lacquers, varnish removers and wood stains.
Acetic acid Acetic acid Acetic acid Acetic acid Acetic acid Acetic acid Acetic acid Acetic acid Acetic acid Acetic acid