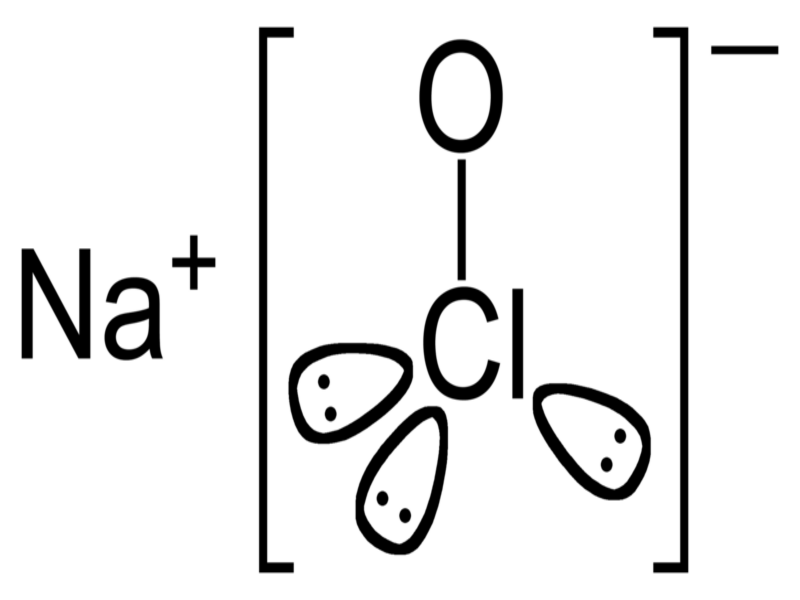
Sodium hypochlorite is a chemical compound that is the sodium salt of hypochlorous acid, with the formula NaOCI or NaCIO, containing a cation (Na) and a hypochlorite anion (OCI- or CIO−).
Heterogeneous reactions of sodium hypochlorite and metals such as zinc proceed slowly to give the metal oxide or hydroxide:
NaOCI Zn → ZnO NaCI
Homogeneous reactions with metal coordination complexes proceed somewhat faster.
Sodium hypochlorite, The anhydride compound is unstable and explosive. It can be crystallized as a pentahydrate NaOCI·5H2O, a pale greenish-yellow solid that is non-explosive and stable when stored under refrigeration. Sodium hypochlorite,
Sodium hypochlorite, Its corrosive properties, its frequent availability, and its reaction products make it a significant safety risk. Sodium hypochlorite, Most commonly produced by mixing liquid bleaches with other cleaning products such as acids or ammonia, produces toxic fumes.
Sodium Hypochlorite is a chlorine compound commonly used as a disinfectant or a bleaching agent. Sodium hypochlorite in a 0.5% w/v solution is called Dakin's solution and is used as an antiseptic to clean infected topical wounds.
Sodium hypochlorite appears as a colorless or slightly yellow aqueous liquid with the odor of household bleach. It is mixed with water.
Sodium hypochlorite is often dissolved in water at various concentrations. Although it exists, solid sodium hypochlorite is not commercially available. Sodium hypochlorite solutions are clear, greenish to yellow liquids with a chlorine odor.
Sodium hypochlorite, There are also many other uses for sodium hypochlorite. In oil production, it is added to the water used to prevent fungal growth that can cause clogging. Sodium hypochlorite, It is used as a sweetener in oil refineries and is also used in the production of chlorinated trisodium phosphate.
Sodium hypochlorite, Sodium hypochlorite, Sodium hypochlorite, Sodium hypochlorite, Sodium hypochlorite, Sodium hypochlorite, Sodium hypochlorite, Sodium hypochlorite, Sodium hypochlorite,