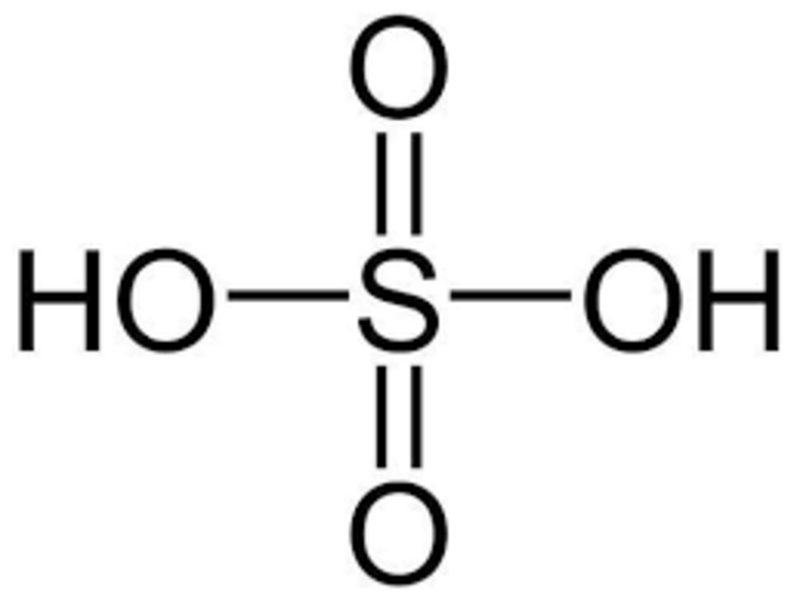
Sulfuric Acid It is a colorless, highly concentrated, strong mineral acid with the chemical formula H2SO4. It was noticed by Cabir Bin Hayyan in the 8th century and is generally produced in concentrated form. Concentrated sulfuric acid contains 96-98% H2SO4 by mass. One of the properties of Sulfuric Acid is that it is a substance close to water. Concentrated sulfuric acid draws water from many organic substances and creates an exothermic reaction. This electrically conducting substance, which produces very high heat when dissolved in water, is known among the public as "battery acid".
Sulfuric acid is produced in industry by the contact method or the lead chamber method. The industrial method used in the production of sulfuric acid is called the "contact process". In the first step, sulfur dioxide is formed by the combustion of sulfur and sulfites.
S8 (g) + 8 O2 (g) ? 8 SO2 (g) (?H° = -2374 kJ/mol)
2 H2S (g) + 3 O2 (g) ? 2 SO2 (g) + 2 H2O (g) (?H° = -1037 kJ/mol)
Sulfuric Acid reacts with more oxygen to form sulfur trioxide, but this reaction is slower. In the contact process, a mixture of sulfur dioxide and oxygen passes through the surface of platinum metal or vanadium oxide catalysts.
Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid Sulfuric Acid